Research
Some of our previous projects include:
Electrochemistry of Reversible Ionic Liquids
Ionic liquids - salts with melting points less than 100°C, low volatilities, are non-flammable and ionically conductive - are useful as electrochemical solvents because of these properties and others. Many have wide electrochemical potential windows which make reactions possible that would not be in aqueous-phase systems, while maintaining high conductivity that traditional organic solvents do not possess. The electrochemical properties of the most popular ionic liquids are well understood, but many new and/or uncommon ionic liquids have yet to be investigated electrochemically.
Reversible ionic liquids, a type of switchable solvent that can be switched from a molecular liquid (with properties like an organic solvent of low viscosity, non-ionic, and relatively non-polar) to a reversible ionic liquid (with properties like an ionic liquid - viscous, ionic, polar) with the introduction of low pressure carbon dioxide (1 atm). The reversible ionic liquid can be returned back to the molecular liquid state with mild heating:
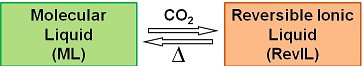
These reversible ionic liquid systems can be utilized for both reactions and separations and hold promise electrochemically. The goal of this project is to investigate the electrochemical properties and stability of the reversible ionic liquids under electrochemical conditions as a function of molecular liquid structure.
The ability of the reversible ionic liquid to perform a switch from the conducting ionic liquid to non-conducting molecular liquid with the addition of heat gives it potential to be applied as a switchable electrolyte to improve battery safety.
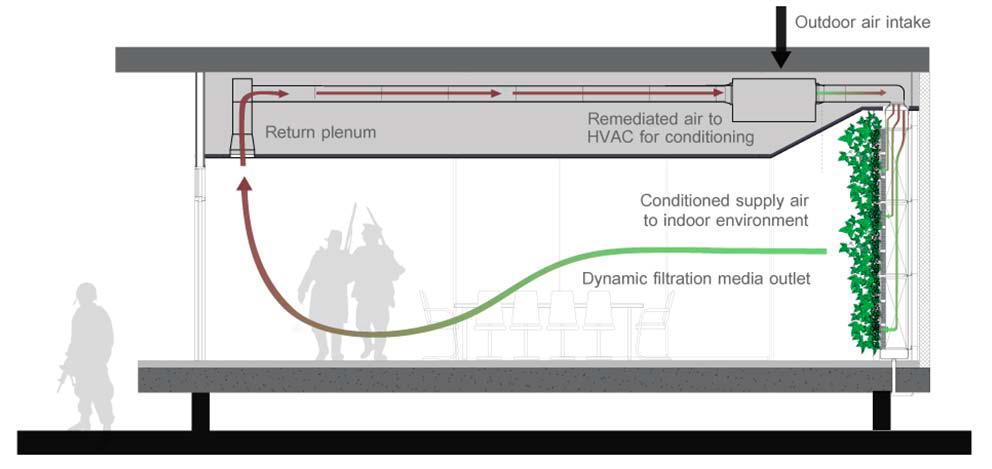
Biowall Project:
The risk of indoor warfare agent exposure is possible due to the intake of outside air through a building's HVAC system. To minimize this risk, the design of a botanically-based air purifying system that can be integrated into an HVAC system to produce clean air is necessary. The design involves the use of ionic liquids tethered to activated carbon in a dynamic filtration media for the capture of warfare agents and other toxins while also supporting a building-integrated active vegetation system.
Fission Product Recovery by Electrodeposition in Ionic Liquids
In U.S., nuclear power plant waste is stored untreated at power plants indefinitely. This waste is composed of unreacted fuel and both non-radioactive and radioactive fission products. The fuel can be recycled and the waste can be segregated by value and half-life for easier handling and storage. Our goal is to selectively deposit metals from waste systems in a recyclable and non-flammable solvent designed for electrochemical systems. Ionic liquids can serve this purpose well. In this project we are identifying and examining promising ionic liquids for the fission products being investigated, the complexing environment of the fission products in ionic liquids, the electrodeposition conditions for recovery of fission products selectively, and the morphology and composition of the deposited fission products.
Below is a scheme for separation and recovery of fission products by electrodeposition in ionic liquids:
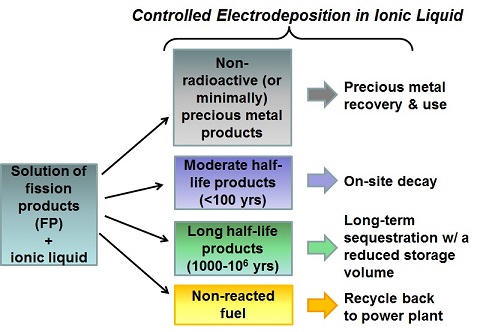
**Non-radioactive fission products and non-radioactive fission product surrogates are used at CCNY. Radioactive fission products are used with collaborators at Brookhaven National Laboratories and Hunter College.**
Electro-Organic Syntheses Paired with Facile Separations
Our goal is to create more sustainable methods of chemical manufacture while maintaining or improving economic viability. Many chemical processes today create large amounts of waste, use large amounts of energy or are inefficient in their use of starting materials. While these processes may have worked well for many years, increased environmental regulations and scarcity of some starting materials lends an opportunity to re-think the way the chemicals are made. Use of new feedstocks like biomass and shale gas also give opportunities for new processes to be developed.
The processes we have proposed utilize electro-organic syntheses paired with facile separations utilizing alternative solvents such as gas-expanded solvents, ionic liquids and switchable solvents. These processes offer the potential to improve product yields, reduce waste products produced and eliminating hazardous steps by pairing electro-organic syntheses with separations utilizing alternative solvents that can be phase-split with introduction of carbon dioxide:
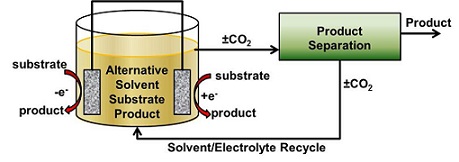
This project requires research and development in:
- Electrocatalysis
- Electrolytes
- Alternative solvents
- Phase equilibria
- Sustainability analysis
- Economic analysis
Funding Sources:
Department of Energy Early Career Award
Nuclear Regulatory Commission
Grove School of Engineering Endowment
CUNY Junior Faculty Research Award in Science and Engineering, Sloan Foundation
PSC-CUNY
MSRDC
ECS Toyota Young Investigator Fellowship
DOE-SBIR
Giner, Inc.
NASA
NSF