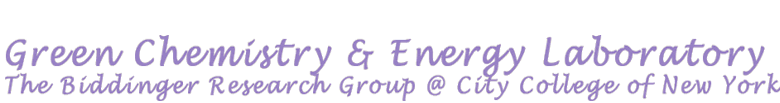Research
We approach green chemistry and energy applications utilizing:
- Electrochemistry
- Catalysis
- Alternative Solvents
- Sustainability Methods
Our interests range from electrocatalysis of biomass-derived materials to development of green and sustainable engineering methods for the production of fine chemicals and pharmaceutical intermediates to the utilization of ionic liquids in batteries and many other interests.
Previous research projects page
Green Chemistry Interests:
Holistically re-evaluate the way we make fine chemicals, biomass-derived chemicals and pharmaceutical intermediates through the development of electrocatalysts, green synthesis and separations methods and use of emerging feedstocks such as biomass and shale gas.
Energy Interests:
Re-examine how we handle alternative energy resources such as biomass-derived fuels and nuclear wastes through the use of electrochemistry, ionic liquids, catalysis and sustainability methods.
Current Ongoing Projects:
Electrochemical Hydrogenation and Hydrogenolysis of Biomass Derived Species
Furfural (FF) is a biomass-derived chemical that is produced in the Quaker process and from the under-utilized C5 stream in a biomass refinery. FF is a platform molecule as it is a reactant in the production of several desirable chemicals. Two chemicals of interest are furfuryl alcohol (FA) and 2-methylfuran (MF), which are considered an adhesive intermediate and biofuel candidate, respectively. FA and MF are produced through the electrochemical hydrogenation and hydrogenolysis (ECH) reactions, respectively, when using FF as the reactant.
In our lab, we investigate the kinetics and the mechanisms for the ECH reactions of FF and HMF. We do this by using batch electrolysis experiments to observe macroscopic trends and by using operando spectroscopic techniques to further investigate the solid-liquid interface. We are also interested in the potential-dependent fouling on copper foil electrodes during ECH of FF in strong acid.

To learn more, please click the links below which lead to some of our recently published works:
https://pubs.rsc.org/en/content/articlelanding/2021/re/d1re00216c
https://doi.org/10.1021/acscatal.9b05531
https://doi.org/10.1016/j.cattod.2018.09.011
https://doi.org/10.1002/ente.201800216
https://doi.org/10.1021/acssuschemeng.6b01314
https://doi.org/10.1021/acs.energyfuels.2c01955
https://doi.org/10.1039/D3GC02222F
CO2 Electroreduction Using Cu-based Catalysts
Carbon dioxide is an abundant waste product and a greenhouse gas. Carbon dioxide can be converted to fuels and chemicals electrochemically, but this process suffers from many challenges. These challenges include poor product selectivity (16 possible products on copper catalysts), reaction competition between hydrogen generation and CO2 reduction (where hydrogen generation becomes a parasitic reaction consuming the electricity intended to be used for CO2ER), catalyst stability, and slow kinetics. Several strategies have been proposed to address these challenges. Our group has identified the catalyst morphology, electrode geometry, and electrolyte composition as key factors that impact both activity and product selectivity. We have found that the electrode geometry significantly affects the current distribution on the electrode surface and consequently, alters the product selectivity and activity. Electrolyte composition is another important factor in CO2ER. Aqueous electrolytes are the most common electrolytes for CO2ER due to their low cost, abundance, eco-friendliness. However, the CO2 solubility in aqueous electrolyte is low. Using ionic liquids additives in aqueous electrolytes is a promising method to enhance the selectivity and activity due to their unique properties such as high CO2 absorption capacity and high stability. We were able to enhance the selectivity of Cu catalysts toward formate (38.7% FE) at -0.92 V vs. RHE by using an ionic liquid additive ([BMIM][NTF2]) in aqueous electrolytes. This enhancement is due to the high hydrophobicity and high CO2 absorption capacity of [BMIM][NTF2]. We also observed that ionic liquid additives affect the local environment at the electrode interface. In-situ spectrometry technique showed us that the presence of additive ions at the electrode surface affect the adsorption of H2O and CO molecules impacting the selectivity and catalytic activity of the CO2ER reaction.

To learn more, please click the links below which lead to some of our recently published works:
https://doi.org/10.1021/acs.iecr.9b03762
https://doi.org/10.1016/j.electacta.2020.136787
https://doi.org/10.1016/j.cej.2021.131303
https://doi.org/10.1021/acscatal.3c00035
Ionic liquid-based electrolytes for lithium metal batteries
Lithium-ion batteries have been the dominate means of rechargeable energy storage since being commercialized in 1991. Rapid advancements in modern technology have increased the demand for high capacity and safe energy storage. For this reason, lithium metal batteries are among the leading candidates to succeed lithium-ion batteries for next generation energy storage. The lithium metal battery has the advantage of a larger theoretical capacity by the means of weight and volume. However, for commercialization of lithium metal batteries, not only concerns of safety with the dendrite formation, but also the poor cycling stability with the high reactivity of lithium metal itself need to be addressed.

In this work, we are approaching these problems by designing Ionic liquid-based electrolyte systems. Ionic liquids have novel, tunable chemical and electrochemical characteristics such as non-flammability, wide electrochemical window, moderate conductivity, and so on. Our goal is to design and understand the ionic liquid-based electrolyte which could operate at the extreme condition found in space, especially toward extremely low temperature, at least -40oC. We are investigating the fundamental physical and electrochemical properties of the ionic liquids, also the interphase between the electrolyte and the electrode surface, and ultimately the relationship of the properties with the safety and the cycle performance.
This project is a collaborative effort funded by NASA. To learn more about the research efforts of our center, click here:
https://batteries-for-space.ccny.cuny.edu/
To learn more, please click the links below which lead to some of our recently published works:
https://doi.org/10.1021/acs.iecr.2c00498
Electrochemical dehydrogenation of liquid organic molecules for hydrogen storage and transport
Hydrogen, a versatile energy carrier and important chemical, holds immense potential for a sustainable and green future. However, hydrogen is the world’s smallest molecule and can easily diffuse, making the storage and transportation of it challenging using the conventional methods such as compressed gas and liquified hydrogen storage. One of the most promising alternatives to store and transport hydrogen is via liquid organic hydrogen carriers (LOHC). LOHC is attractive method for hydrogen storage and transport due to its chemical properties, which allow using existing infrastructures. Hydrogen can be “loaded” to an organic molecule through hydrogenation process and be “released” through dehydrogenation process. Electrochemical process for these two processes offers an opportunity to utilize renewable electricity for storing and transporting hydrogen and therefore accounting for low carbon footprint in comparison to the existing methods.
Our group focuses on studying the electrochemical dehydrogenation (ECD) reactions to release hydrogen which are often more challenging than electrochemical hydrogenation reactions. The goal of our group is to study the kinetics and reaction mechanisms for ECD of various LOHC molecules.
To learn more, please click the link below to read our recently published work:
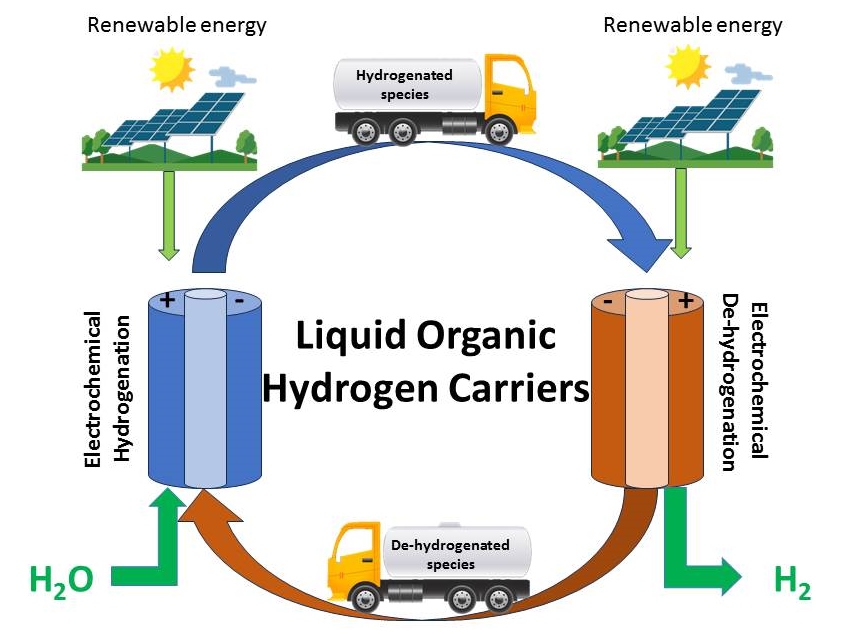
Polypeptide Complex Coacervates for CO2 Capture
Complex coacervation is a form of liquid-liquid phase separation that can be formed from oppositely charge polyelectrolytes (PEs). They have gained interest for their ability to compartmentalize molecules. Changes in pH act as a stimulus by protonating or deprotonating weak PEs, thus strengthening or weakening the electrostatic interactions that drive coacervation. In our lab we study coacervates of Poly(L-lysine hydrochloride), (PLys) and Poly(D,L-glutamic acid sodium salt), (PGlu). This work investigates CO2 as an external stimulus to a complex coacervate system in an alkaline environment. The addition of CO2 will reduce the pH and produce bicarbonate and carbonic acid. NaOH is also used to deprotonate the ammonium group on the polycation of the coacervate system, poly(L-lysine hydrochloride). This newly formed amine can react with CO2, acting as a secondary source of bicarbonate. We explore how reversibility, molecular speciation, and accompanying pH changes influence behavior.
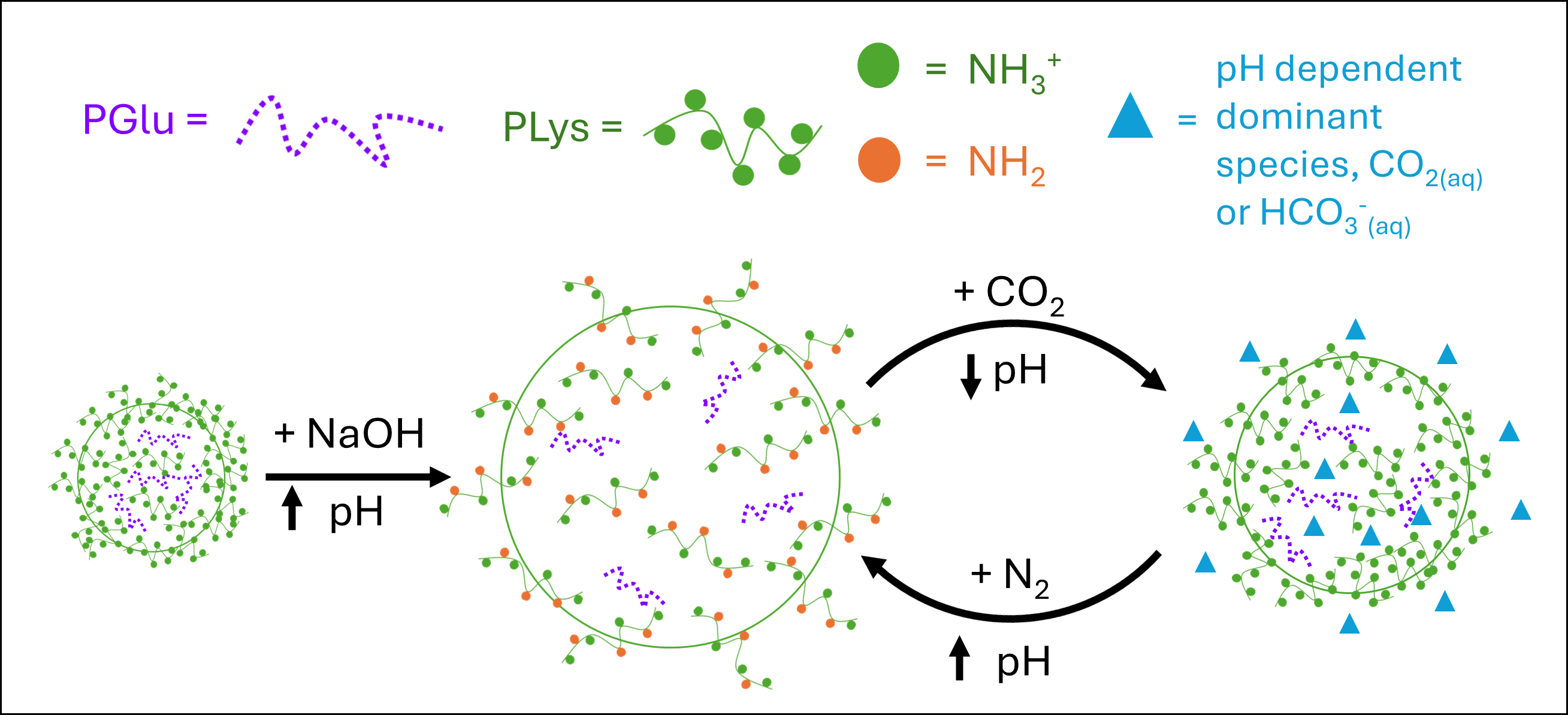
Power Grid and Electrical Infrastructure Optimization to Account for Electrification of Energy-Intensive Processes
As we continue to progress toward global climate goals, there is an increasing interest in decarbonizing hard-to-abate industries. The chemical industry produces over 7% of global greenhouse gas emissions, primarily due to the prominence of fossil fuels as both feedstock and energy sources in major chemical production processes. The electrification of energy-intensive processes through electric heating and electrochemical pathways is the most prominent path to decarbonizing the chemical manufacturing industry. However, this would cause a significant increase in electrical demand, underscoring the importance of collaborative research efforts between the chemical and electrical industries.
We aim to develop materials for the decarbonization of the chemical industry while accounting for and optimizing its impact on the electrical grid, particularly focusing on the role of hydrogen in both industries.
Funding Sources:
Department of Energy Early Career Award
Nuclear Regulatory Commission
Grove School of Engineering Endowment
CUNY Junior Faculty Research Award in Science and Engineering, Sloan Foundation
PSC-CUNY
MSRDC
ECS Toyota Young Investigator Fellowship
DOE-SBIR
DOE ARPA-E
Giner, Inc.
NASA
NSF
NSF ECO-CBET
Sloan Foundation
DOE-FAIR
New York State
Turnover Labs